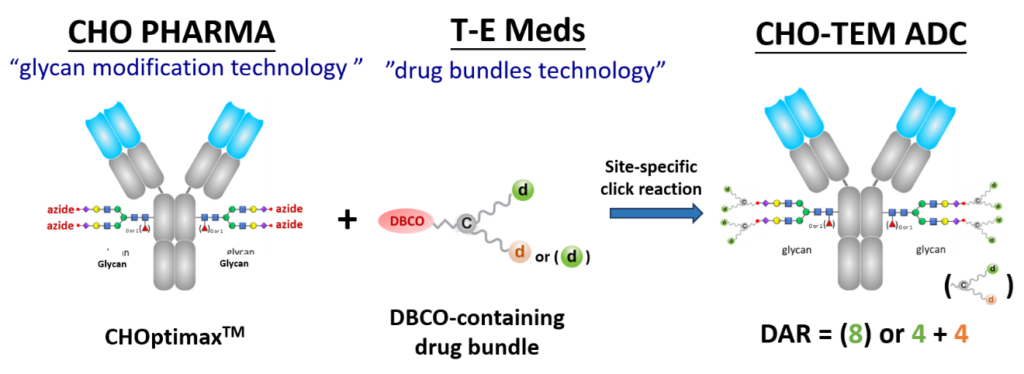
T-E Meds possesses an innovative “drug bundle” technology that facilitates the packaging of multiple drug molecules, whether of the same or different types, into a bundled configuration equipped with a click chemistry functional group, such as DBCO.
These drug bundles, predominantly synthesized through solid-phase peptide synthesis, comprise hydrophilic amino acids and PEGylated amino acids, with varying ethylene glycol unit counts, allowing for tailored hydrophilicity.
We are thrilled to establish a strategic business alliance with CHO Pharma Inc., the collaboration aims to leverage our complementary technologies in the development of novel CHO-TEM ADC products.
CHO Pharma has a unique “glycan modification” technology for precise alteration the glycan structure of IgG Fc. This process requires only one glycosyltransferase for cleaving off carbohydrate chains, leaving behind a monosaccharide (with or without fucose), and then ligating a prepared oligosaccharide.
Notably, the oligosaccharide consists of 11 linked monosaccharides, including two branching stretches of 5-member oligosaccharides, with the terminal sialic acid already conjugated with an azido group.
When mixed in an aqueous solution without the need for organic solvents, the drug bundles and glycan-modified antibodies exhibit rapid reactivity, achieving a conjugation rate exceeding 95%.
The resulting ADC products boast a high Drug-to-Antibody Ratio (DAR) of 8, consisting of eight drug molecules of the same type or four each of two different types. These ADCs have demonstrated the excellent efficacy in killing tumor cells in vivo cytotoxicity experiments and exhibited stability, with no drug molecule or drug bundle shedding observed in human serum and plasma in vitro.
In summary, CHO-TEM ADC technology surpasses other ADC technologies, including those by Synaffix, in producing ADC candidates with higher DAR using a simplified process.
Here is an example of the CHO-TEM ADC technology.