T-E Meds possesses an innovative “drug bundle” technology that facilitates the packaging of multiple drug molecules, whether of the same or different types, into a bundled configuration equipped with a click chemistry functional group, such as DBCO.
These drug bundles, predominantly synthesized through solid-phase peptide synthesis, comprise hydrophilic amino acids and PEGylated amino acids, with varying ethylene glycol unit counts, allowing for tailored hydrophilicity.
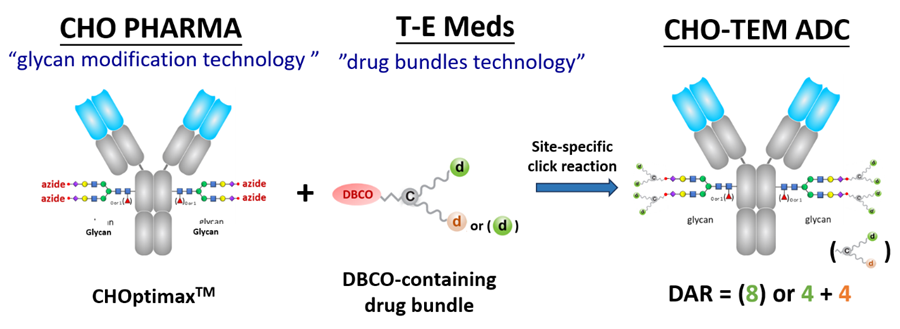
We are thrilled to establish a strategic business alliance with CHO Pharma Inc., the collaboration aims to leverage our complementary technologies in the development of novel CHO-TEM ADC products.
CHO Pharma has a unique “glycan modification” technology for precise alteration the glycan structure of IgG Fc. This process requires only one glycosyltransferase for cleaving off carbohydrate chains, leaving behind a monosaccharide (with or without fucose), and then ligating a prepared oligosaccharide.
Notably, the oligosaccharide consists of 11 linked monosaccharides, including two branching stretches of 5-member oligosaccharides, with the terminal sialic acid already conjugated with an azido group.
When mixed in an aqueous solution without the need for organic solvents, the drug bundles and glycan-modified antibodies exhibit rapid reactivity, achieving a conjugation rate exceeding 95%.
These constituted antibodies encompass whole IgG molecules, targeting well-established carcinogenic antigens and are site-specifically conjugated to four drug bundles, each carrying 2-4 cytotoxic molecules.
Notably, CHO-TEM ADCs exhibit homogeneity with high DAR of 8, 12 or 16, showing superior in vitro and in vivo stabilities.
Here is an example of the CHO-TEM ADC technology.
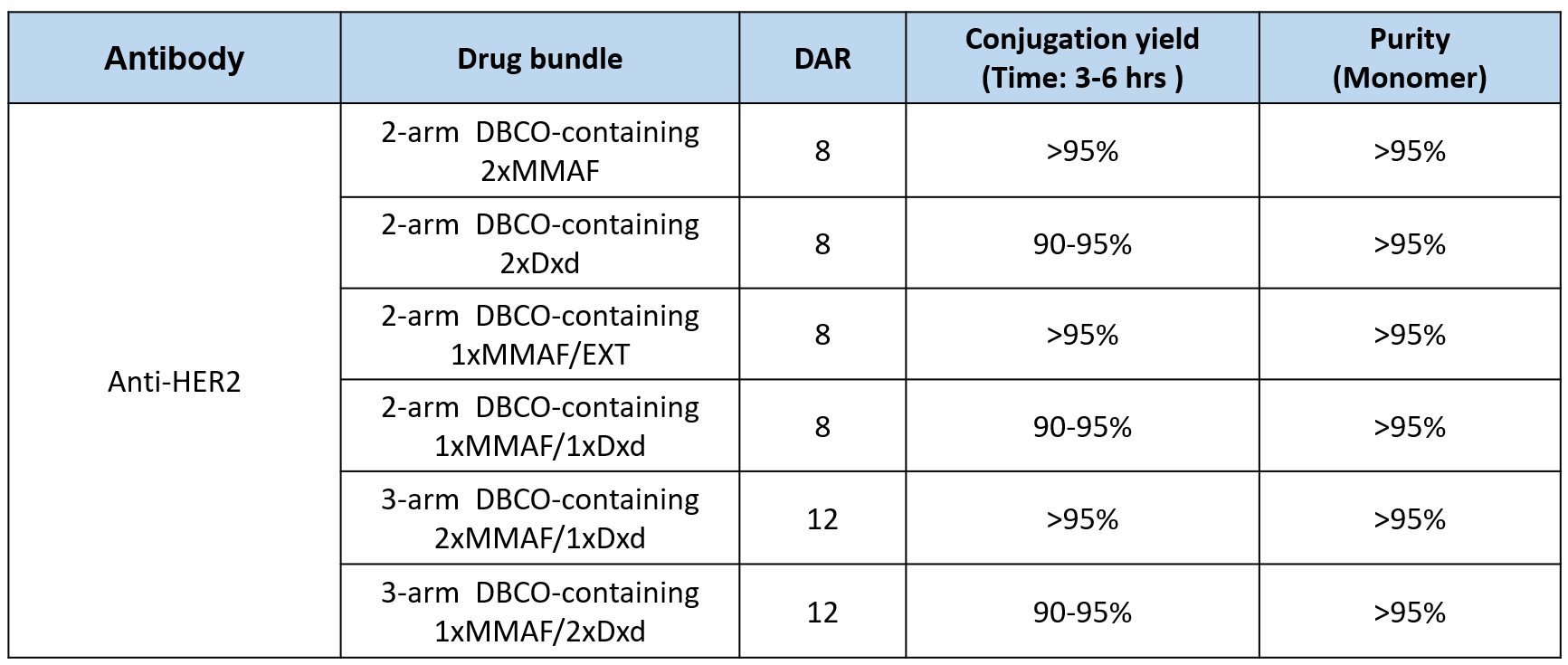
Leveraging this platform, we can generate a series of CHO-TEM ADCs capable of recognizing different cancer antigens and carrying various cytotoxic payloads (as listed in the table below). These ADCs achieve DAR values of 8 or 12, with a high conjugation yield (>90%), and the final ADC products exhibit a purity exceeding 95%.
The drug molecules engineered using the CHO-TEM ADC platform have successfully overcome critical bottlenecks in ADC drug development, exhibiting five key advantages that significantly enhance their market competitiveness:
- Produce homogenized ADC: Using site-specific conjugation to attach drug bundles to antibodies, enabling the production of high-purity and homogenized ADC drugs.
- Simple and Efficient Process: No need for non-natural amino acids or enzyme catalysis.
- High Drug-to-Antibody Ratio (DAR): Enables the production of ADC drugs with a high DAR, further enhancing therapeutic efficacy.
- Good Solubility and Stability: Through adjustable multi-arm linker designs, the resulting ADC drugs exhibit excellent solubility and stability, improving pharmacokinetic performance and reducing toxicity risks.
- Dual-Payload Capability: Successfully overcomes the technical challenge of dual payloads, enabling ADC drugs to carry two different drugs and providing new opportunities for the treatment of drug-resistant and heterogeneous tumors.
